The Electron Sea Model Is Used to Describe Bonding in
Or energystate than the unbonde. In terms of this model all the metal atoms contribute their ___ electrons to form an electron sea so that the electrons are ___ or shared among all the atoms.
What Is Metallic Bonding Using The Electron Sea Model To Explain It
An introduction to the Sea of Electrons Model used to describe bonding between metals.

. Electrons move freely. Explain and use the VSEPR Valence Shell Electron Repulsion Theory to predict shapes and polarity of molecules. The electron sea model is a model of metallic bonding in which cations are considered to be fixed points within a mobile sea of electrons.
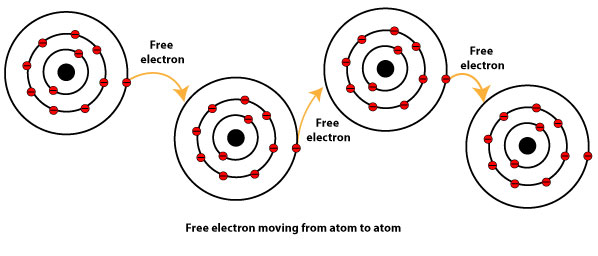
These electrons are then free to move about the mostly vacant outer orbits of all metal atoms. How are they the same. The metal is held together by the attraction between the metal ___ and the sea of electrons.
Ball and stick model d. This gives the metallic bond distinct properties in contrast to ionic and covalent bonds. Each atom contributes its valence electrons to a region surrounding the atoms.
Use the electron-sea model to explain metallic properties. This model proposes that all the metal atoms in a metallic solid contribute their valence electrons to form a sea of electrons. A metallic bond is a bond between two metal atoms.
The simplest model used to describe the bonding in metals is the electron-sea model. What is a sea of Delocalised electrons. Valence shell electron pair repulsion theory b.
The loosely bonded electrons can move freely in these orbitals to delocalize through out the whole crystal. Metallic bonding results from the transfer of valence electrons. What is the electron sea model.
Two or more metals can combine to form alloys of variable composition by sharing their valence electrons. Describe the electron sea model of metallic bonding. It is the simplest metal bonding model.
The valence electrons of the bonded atoms are shared in an electron sea. The electron sea model for. Describe the electron-sea model of metallic bonding.
The atoms are arranged so that each sodium atom is surrounded by eight other. Electron sea model Feedback The correct answer is. The bonded state is usually a electrons.
How is the electron sea model of metallic bonding different from the band theory. The model used to describe and explain the bonding and arrangement of atoms in a solid metal is the Select one. Since these lattices do not fracture easily metals are said to be highly ductile.
The model of metallic bonding where electrons float free in a sea of electrons around metal atoms. Therefore when metals are beaten with a hammer the rigid lattice is deformed and not fractured. The ability of electrons to move freely among bonded metal atoms is known as the sea of electrons bonding model.
Atoms form compounds by B. Metallic bonding is known as the electron-sea model. The similar half filled orbitals of adjacent metals can overlap with each other in metal crystal.
The model shows a portion of the crystal structure of solid sodium. The crystal structure of atoms in metallic bonds allows the metal to maintain a constant pattern when force is applied. An explanation of how this influences the characteristics of metals i.
Metallic bonding results from the sharing of valence electrons. Chemical Bonding Chemical Bonds A. Delocalized electrons also exist in the structure of solid metals.
It is the most complicated metal bonding model. Metallic structure consists of aligned positive ions cations in a sea of delocalized electrons. Learn about metallic bonding with an explanation of the unique properties of metals and understand why.
Electrons all have approximately the same energy. Give at least one similarity and one difference between the models. The sea of free electrons in metallic bonds allow the atoms to move when stressed without changing the properties of the substance.
Compared to nonmetals metals have low ionization energies allowing them to lose valence electrons easily. Electrons move among orbitals of different energies. Find step-by-step Chemistry solutions and your answer to the following textbook question.
The electrons are attracted to specific. Select all the statements that correctly describe the electron-sea model of metallic bonding. Explanation of the electron sea model Because of low ionization energy the valence electrons of metals are loosely bonded within the atom.
Describe the electron-sea model of metallic bonding. Metallic bonds and most of their properties can be explained using the simple electron sea model. A model of metallic bonding in which cations are considered to be fixed points within a mobile sea of electrons.
The electrons are delocalized. It is generally viewed as the result of mutual sharing of many electrons by atoms. This is why metals can be beaten into thin sheets.
Describe the electron-sea model of metallic bonding. In the case of metals the sea of electrons in the metallic bond enables the deformation of the lattice. With this behavior unique to metallic bonding metals exhibit many properties that.
Check all the boxes that describe the electron sea model.

Metallic Bonding And The Electron Sea Model Electrical Conductivity Basic Introduction Youtube
Properties Of Metallic Bonding Ppt Download

Bonding In Metals The Electron Sea Model Introduction To Chemistry
No comments for "The Electron Sea Model Is Used to Describe Bonding in"
Post a Comment